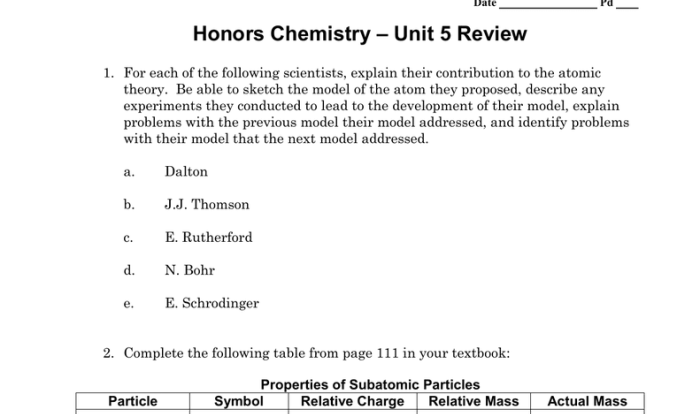
Welcome to the realm of chemical equations, where understanding their intricate balance is crucial. This exploration delves into the student exploration balancing chemical equations answer key, providing a comprehensive guide that empowers learners with the knowledge and tools to master this fundamental chemistry concept.
Through engaging activities, simulations, and step-by-step guidance, this guide unlocks the secrets of balancing chemical equations, fostering a deeper comprehension of chemical reactions and their significance.
Introduction
Chemical equations are symbolic representations of chemical reactions that show the chemical formulas of the reactants and products involved in the reaction. Balancing chemical equations is a fundamental skill in chemistry that ensures that the number of atoms of each element is the same on both sides of the equation.
This ensures that the law of conservation of mass is upheld, which states that mass cannot be created or destroyed in a chemical reaction.
Student Exploration
Student exploration plays a crucial role in understanding chemical equations. Hands-on activities and simulations can help students visualize the chemical reactions and the changes that occur during the reaction. This experiential learning approach enhances comprehension and makes the learning process more engaging.
Balancing Chemical Equations
- Identify the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients in front of the chemical formulas to make the number of atoms of each element equal on both sides.
- Check the equation again to ensure that it is balanced.
Example:
Unbalanced equation: CH 4+ 2O 2→ CO 2+ H 2O
Balanced equation: CH 4+ 2O 2→ CO 2+ 2H 2O
Practice and Application
Practice problems and scenarios can help students apply their understanding of balancing chemical equations. These exercises can range from simple equations to more complex reactions. Students can also engage in peer review by sharing their solutions and providing feedback on each other’s work.
Assessment and Feedback, Student exploration balancing chemical equations answer key
Assessing student understanding of balancing chemical equations can be done through quizzes, tests, or problem-solving tasks. Constructive feedback should be provided to students to help them improve their performance. Feedback should focus on the accuracy of the balanced equation, the steps taken to balance it, and any common errors that were made.
Detailed FAQs: Student Exploration Balancing Chemical Equations Answer Key
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side, accurately representing the conservation of mass in chemical reactions.
How can student exploration enhance the understanding of chemical equations?
Student exploration through hands-on activities and simulations allows learners to actively engage with the concepts of chemical equations, fostering a deeper comprehension of the underlying principles and their practical applications.
What are the key steps involved in balancing chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula until the number of atoms of each element is equal on both sides of the equation.