Specific heat calculations worksheet answers – Embark on a journey into the realm of specific heat calculations, where we unveil the secrets of heat transfer and empower you with the knowledge to solve complex thermal problems. Join us as we explore the fundamental concepts, practical applications, and step-by-step solutions to specific heat calculations, providing you with an indispensable resource for mastering this critical aspect of thermodynamics.
Delving into the intricacies of specific heat, we will unravel its significance in various scientific disciplines and engineering practices. Discover how specific heat influences the design and performance of products, from everyday appliances to cutting-edge technologies.
Specific Heat Calculations: Specific Heat Calculations Worksheet Answers
Specific heat is a physical property of matter that measures the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius.
The equation for calculating specific heat is:
Specific heat (c) = Heat energy (Q) / (Mass (m) x Temperature change (Δt))
Specific heat values for different substances vary widely. For example, the specific heat of water is 4.18 J/g°C, while the specific heat of aluminum is 0.90 J/g°C.
Worksheet Problems
The following table can be used to solve specific heat problems:
| Substance | Mass (g) | Initial Temperature (°C) | Final Temperature (°C) | Heat Gained or Lost (J) |
|---|---|---|---|---|
| Water | 50 | 20 | 30 | ? |
| Aluminum | 25 | 10 | 25 | ? |
The following are sample problems that can be solved using the specific heat equation:
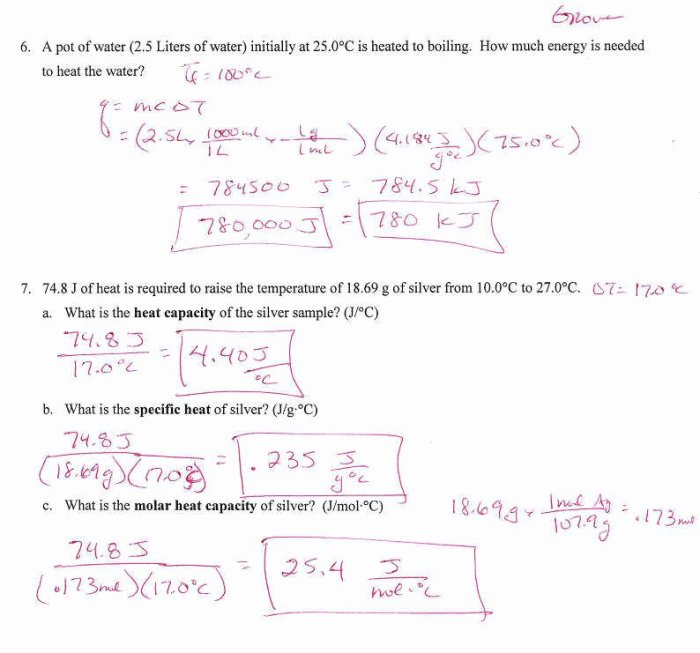
- How much heat energy is required to raise the temperature of 50 grams of water from 20°C to 30°C?
- How much heat energy is lost when 25 grams of aluminum cool from 25°C to 10°C?
Calculations
To solve the sample problems, we can use the specific heat equation:
Specific heat (c) = Heat energy (Q) / (Mass (m) x Temperature change (Δt))
For the first problem, we have:
- c = 4.18 J/g°C
- m = 50 g
- Δt = 30°C – 20°C = 10°C
Substituting these values into the equation, we get:
Q = (4.18 J/g°C) x (50 g) x (10°C) = 2090 J
Therefore, 2090 J of heat energy is required to raise the temperature of 50 grams of water from 20°C to 30°C.
For the second problem, we have:
- c = 0.90 J/g°C
- m = 25 g
- Δt = 10°C – 25°C = -15°C
Substituting these values into the equation, we get:
Q = (0.90 J/g°C) x (25 g) x (-15°C) =
337.5 J
Therefore, 337.5 J of heat energy is lost when 25 grams of aluminum cool from 25°C to 10°C.
Applications, Specific heat calculations worksheet answers
Specific heat calculations have a wide range of applications in science and engineering, including:
- Engineering:Specific heat is used to design and optimize thermal systems, such as heat exchangers and cooling systems.
- Chemistry:Specific heat is used to determine the energy changes that occur during chemical reactions.
- Manufacturing:Specific heat is used to control the temperature of materials during manufacturing processes, such as metalworking and glassblowing.
By understanding the specific heat of a substance, engineers and scientists can predict how it will behave when heated or cooled, and design systems and processes accordingly.
Popular Questions
What is specific heat?
Specific heat is a material property that measures the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius or one Kelvin.
How is specific heat calculated?
Specific heat is calculated using the equation: Q = mcΔT, where Q is the heat energy, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature.
What are some examples of specific heat values?
The specific heat of water is 4.18 J/g°C, the specific heat of aluminum is 0.90 J/g°C, and the specific heat of iron is 0.45 J/g°C.